CDMO
Contract development and manufacturing
Our integrated scientific services, from early discovery to pre-clinical studies.

INFLUENZA
Innovative vaccine development
Developments in Influenza vaccines paving new paths in medicine and healthcare

INNOVATION
R&D activities
Through continuous research and development, we play a key role in developing numerous new technologies and therapies. Contract R&D and manufacturing organization.


Who we are
Fluart Innovative Vaccines Ltd. is Hungary’s only pharmaceutical, biotechnology, and life sciences research company that has been pioneering influenza vaccine research and production since 1991. Our company is committed to healthcare innovation and contributes to the wide availability of vaccines. As a Contract Development and Manufacturing Organization (CDMO), we also offer integrated scientific services.
What we do
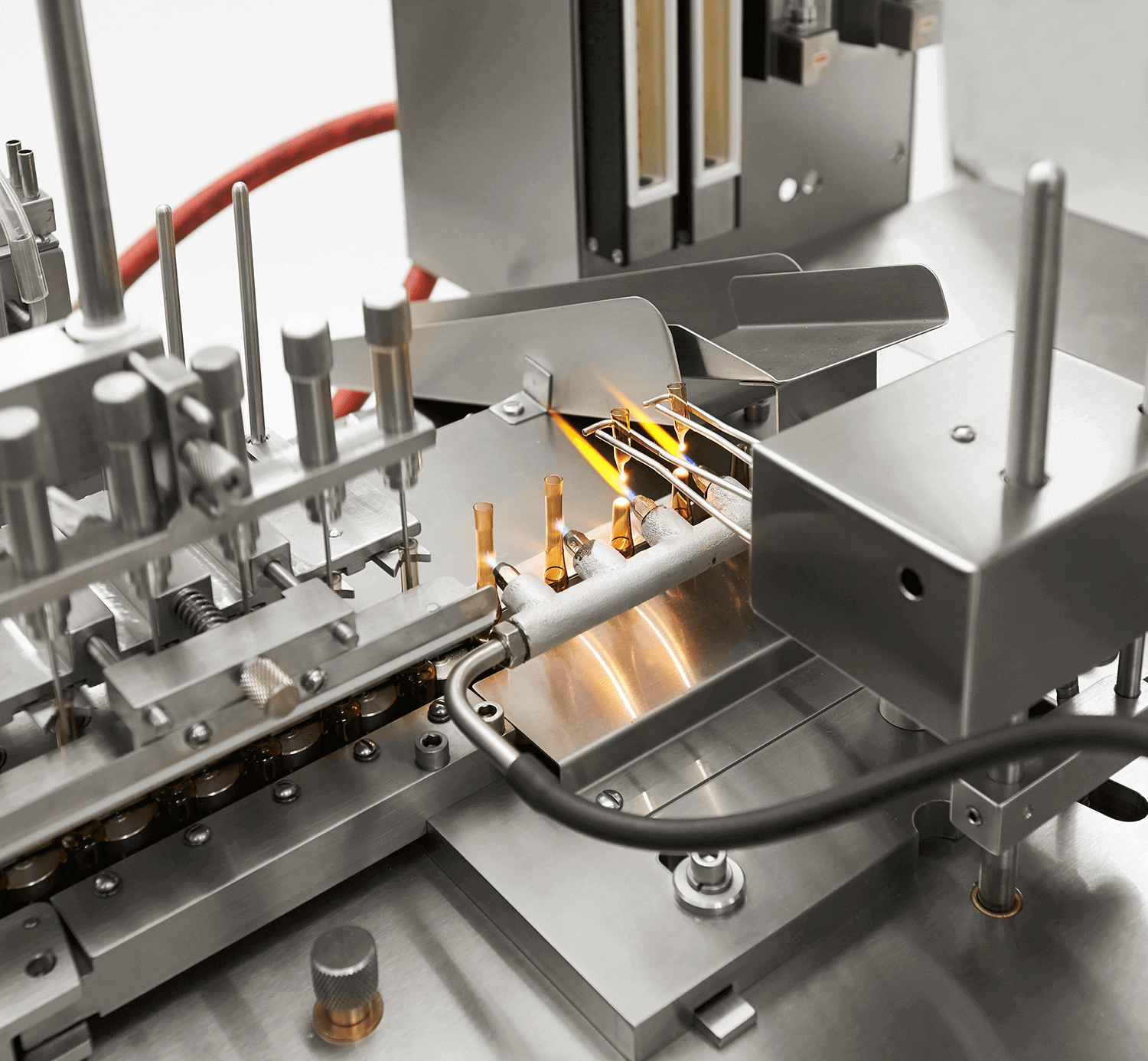
We are a leading player in the Hungarian vaccine market, particularly in the research, development, and production of influenza vaccines. Through our CDMO services, we provide reliable support from clinical trials to commercial manufacturing. Our developments also encompass bacteriophage research, animal health, and the food industry. Our production follows GMP regulations, ensuring seamless scale-up.

CDMO services
We are a strategic and reliable partner in pharmaceutical product programs, whether it involves clinical trials of small-scale materials or the manufacturing of commercially launched products.

Research and Development
In addition to our forward-looking achievements in vaccine research and development, we successfully respond to the needs of various industrial sectors with our development projects and comprehensive services. These extend to bacteriophage research, animal health, and the food industry.

Our Story in numbers
Behind every number lies real achievements and commitment, demonstrating our expertise and reliability. We are proud to contribute to the advancement of preventive medicine and the protection of human health with innovative solutions. Measured in numbers, but lived through human connections – this is our story.
33+ years of experience
in the development and manufacturing of influenza vaccines
35+ million
influenza vaccines produced
1800+ square meters
R&D and manufacturing infrastructure
12 publications
in scientific journals
50+ partners
in scientific and professional collaborations
Industries
We cater to the needs of numerous industries, including pharmaceutical, biotechnology, food, animal health, and food manufacturing companies conducting research projects. Through our innovative, comprehensive research and development services and projects, we meet the demands of these industries.
Professional recognition
Our company is internationally recognized as a leading manufacturer. We are regular invited participants and speakers at WHO scientific events, maintaining continuous contact and collaboration with international organizations in the field (CDC, WHO, ECDC, NIH, EMA, NIBSC).
Contact us
We are happy to answer any questions you may have, whether it’s about our CDMO services, products, or research projects.
CAREER
Are you looking to establish a career in the pharmaceutical industry?
Join our dedicated team, pioneering research in collaboration with domestic and international clients. Explore exciting opportunities in the development, manufacturing, and research of human vaccines. Join us in advancing projects that bring about change in the pharmaceutical industry.



