CDMO
Pharmaceutical contract services
Contract development and manufacturing, contract manufacturing services for all stages of drug development.
A pharmaceutical company with over 33 years of experience in innovative pharmaceuticals, specialising in the production, research and development of human vaccines.
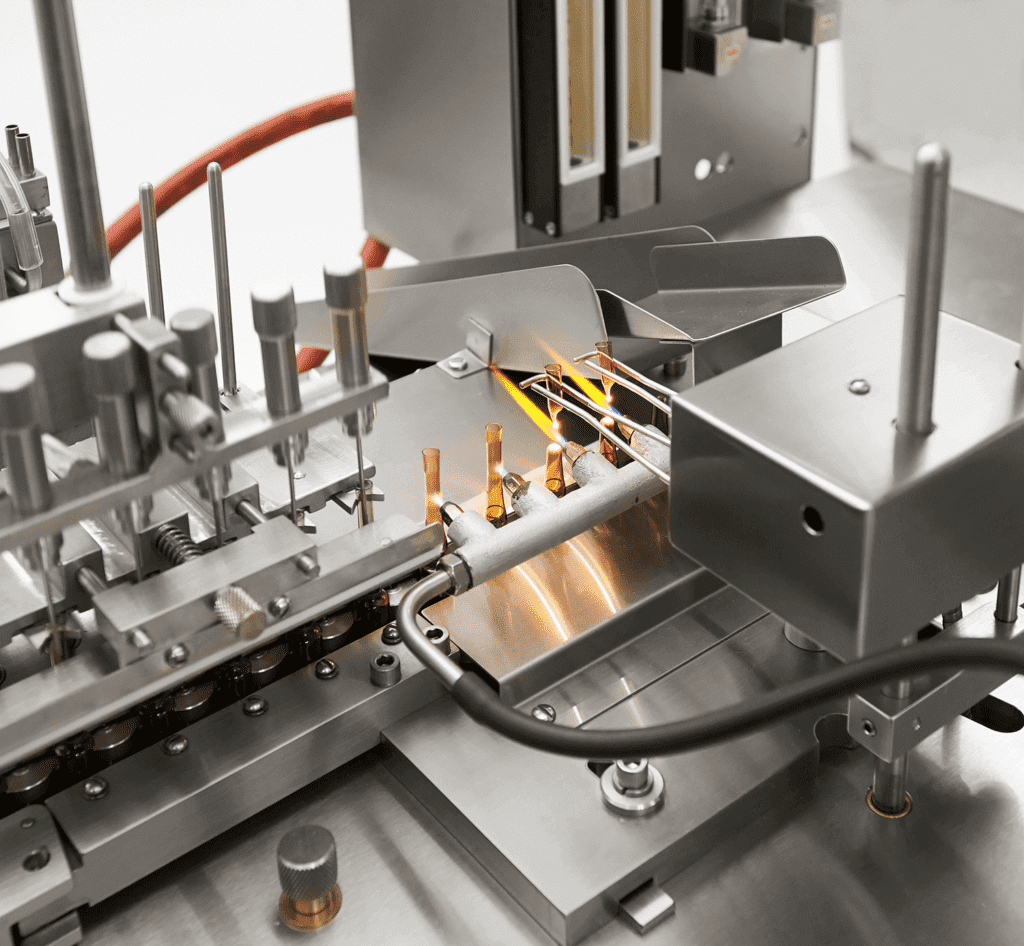
Our plants are capable of producing small volume biological products and APIs under GMP conditions, as well as pre-clinical and clinical trial samples. Our company undertakes GMP framework-based contract development and manufacturing (CDMO) projects for potential partners, including manufacturing activities, quality control, logistics and full documentation.

Overview of capabilities
CDMO Services
We are committed to supporting the needs of biotech, pharmaceutical and life science companies with our proven end-to-end CDMO capabilities and technical expertise.


CDMO Services
Development service

CDMO Services
Manufacturing service


CDMO service
Analytical, quality control and quality assurance services

FLUART CDMO
Focus on what you do best
Our expertise and wide range of services relieve our customers of the burden of manufacturing tasks, allowing them to focus all their efforts on growing the market, generating demand and driving new product growth through marketing and distribution. We work closely with our customers, representing those who put human vaccines first.
Let’s work together for a healthier future!
Whether it’s developing an idea or improving an existing solution, we have the expertise and resources to support you every step of the way. Fluart is not just a service provider, but an ally, fully committed to partnership and mutual success.

